Home › Forums › Health, Biology, and Science Forum › Science and Technology Forum › What is Geometrical Isomerism?
Tagged: atoms, cis isomer, cis-2-butene, cis-9-octadecenoic acid, cis-butenedioic acid, cis-trans isomerism, cis-trans isomerism definition, cis-trans isomers, cyclic structure, define cis-trans isomerism, define geometrical isomerism, definition of geometrical isomerism, double bond, elaidic acid, examples of geometrical isomerism, fumaric acid, fused ring systems, geometric isomerism, geometrical isomerism, geometrical isomers, heterocyclic, homocyclic, isomeric 2-butenes, isomerism, maleic acid, methyl groups, oleic acid, optically active, organic chemistry, restricted rotation, single bond, stable configurations, trans isomer, trans-2-butene, trans-9-octadecenoic acid, trans-butenedioic acid, type of stereoisomerism
- This topic has 1 reply, 2 voices, and was last updated 10 years, 6 months ago by
 Dr. Aditya Sardana.
Dr. Aditya Sardana.
- AuthorPosts
- 15/08/2015 at 6:16 pm #4156
Alice
ParticipantWhat is geometrical isomerism? Define geometrical isomerism. Give the definition of geometrical isomerism along with examples of geometrical isomerism.
- 20/08/2015 at 3:54 pm #4166
 Dr. Aditya SardanaKeymaster
Dr. Aditya SardanaKeymasterGeometrical isomerism, also known as cis-trans isomerism, is a type of stereoisomerism exhibited by the compounds having the same structure but different configurations. Geometrical or geometric isomerism is shown by a wide variety of molecules that can exist in different stable configurations; the reason for this ability being the presence of a double bond (C=C, C=N, N=N), a cyclic structure (homocyclic, heterocyclic, fused ring systems), or restricted rotation about a single bond. Molecules showing geometrical isomerism are called geometrical isomers or cis-trans isomers. Geometrical isomers may or may not be optically active.
Examples of geometrical isomerism (cis-trans isomerism) include isomeric 2-butenes (cis-2-butene and trans-2-butene), maleic acid (cis-butenedioic acid) and fumaric acid (trans-butenedioic acid), cis-9-octadecenoic acid (oleic acid) and trans-9-octadecenoic acid (elaidic acid), etc.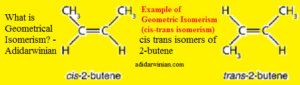
The cis isomer (here, cis-2-butene) has similar (or identical) atoms or groups (here, methyl groups) on the same side, whereas the trans isomer (here, trans-2-butene) has similar (or identical) atoms or groups (here, methyl groups) on the opposite side.Related Posts
- AuthorPosts
- You must be logged in to reply to this topic.
