Home › Forums › Health, Biology, and Science Forum › Science and Technology Forum › What is Geometrical Isomerism? › Reply To: What is Geometrical Isomerism?
Geometrical isomerism, also known as cis-trans isomerism, is a type of stereoisomerism exhibited by the compounds having the same structure but different configurations. Geometrical or geometric isomerism is shown by a wide variety of molecules that can exist in different stable configurations; the reason for this ability being the presence of a double bond (C=C, C=N, N=N), a cyclic structure (homocyclic, heterocyclic, fused ring systems), or restricted rotation about a single bond. Molecules showing geometrical isomerism are called geometrical isomers or cis-trans isomers. Geometrical isomers may or may not be optically active.
Examples of geometrical isomerism (cis-trans isomerism) include isomeric 2-butenes (cis-2-butene and trans-2-butene), maleic acid (cis-butenedioic acid) and fumaric acid (trans-butenedioic acid), cis-9-octadecenoic acid (oleic acid) and trans-9-octadecenoic acid (elaidic acid), etc.
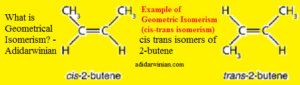
The cis isomer (here, cis-2-butene) has similar (or identical) atoms or groups (here, methyl groups) on the same side, whereas the trans isomer (here, trans-2-butene) has similar (or identical) atoms or groups (here, methyl groups) on the opposite side.
Related Posts
